Copper and Zinc in Living Systems
Tracing element pathways and processes of Cu, and Zn in the geosphere and biosphere: precision measurement with isotopic tools.
Copper and zinc play critical roles in biochemical and geochemical processes. Disruptions in the delicate balance among these elements in living systems are implicated in diseases such as Alzheimer’s, Parkinson’s, and cancer. Often these elements interact in cooperative or antagonistic fashion in biogeochemical processes. The challenge is to gain insights into the physical and chemical mechanisms affecting these elements in living systems and thus understand how these metals are processed in the geosphere and biosphere. Many analytical methods rely on quantification of amount of element, but these data can be ambiguous and do not provide the detail needed to follow an atom through a system. Knowledge of isotopic composition of the element adds a dimension to the data to elucidate source(s) of the element to the system and physical or chemical processes responsible for incorporation and translocation of the element. This is because isotopes of an element contain the same number of protons and different numbers of neutrons. The same number of protons means that neutral atoms have the same number of electrons and participate in chemical reactions in a similar manner. However, different numbers of neutrons result in different masses and this impacts kinetic reaction rates and thermodynamic equilibria of different isotopes. Careful quantification of the redistribution of an element’s isotopes provides valuable insights into processes on an atomic scale. Our goal is to understand why changes in the isotopic composition of Cu and Zn occur and exploit this knowledge to understand the role of these elements in biogeochemical interactions. The objectives of the research program will be realized by (1) development and deployment of stable isotope abundance measurements to uncover the distribution and variability of isotopic abundances in biologically significant systems; (2) identification of critical receptors and interactions where changes in isotopic composition occur; and (3) understanding physical and chemical processes responsible for isotopic variations integrating both experimental and theoretical approaches.
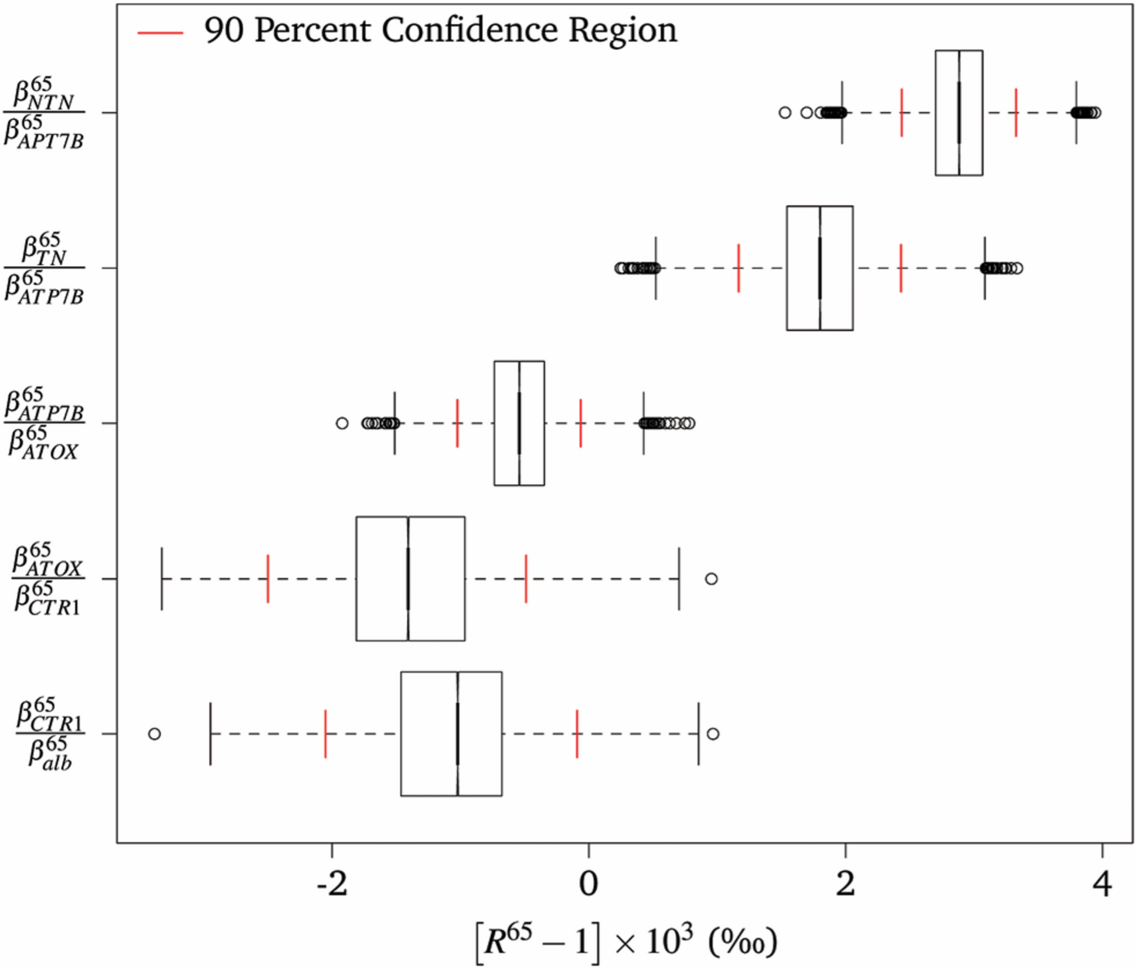
Alex Tennant (MSc, 2015-2017) developed a model to calculate reduced partition function ratios to predict the redistribution of the isotopes of copper as the element is taken up by liver cells and transported via a series of protein interactions to be loaded into blood serum. Alex identified that the transfer of copper from ATP7B to ceruloplasmin had the most significant effect on the copper isotopic composition of the blood serum. The opportunity to apply theoretical models to actual measurements of individual copper-containing proteins remains an active area of research.
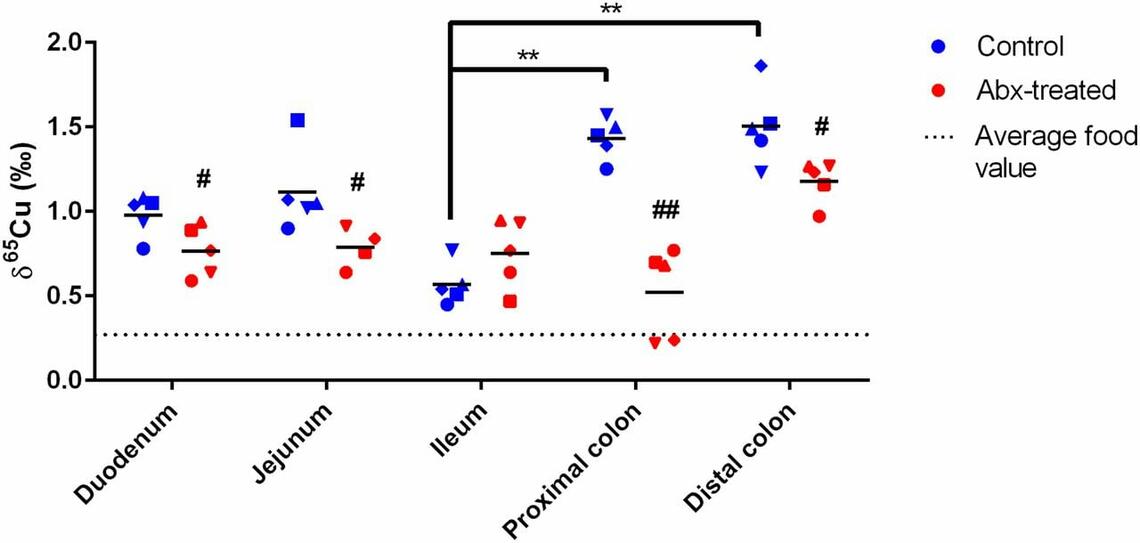
Kerri Miller (PhD, 2013-2018) identified a significant change in the isotopic composition of copper in the gut of mice that were treated with antibiotics. A significant difference, ∼1.0 ‰, in copper isotope abundances was measured in the proximal colon of antibiotic-treated mice. The changes in copper isotopic composition in the colon are accompanied by changes in copper transporters. Both CTR1, a copper importer, and ATP7A, a copper transporter across membranes, were significantly down-regulated in the colon of antibiotic-treated mice. This study demonstrated that isotope abundance measurements of metals can be used as an indicator of changes in metabolic processes in vivo. These measurements revealed a host-microbial interaction in the GI tract involved in the regulation of copper transport.
Regional distribution of Cu isotopes (𝛿65Cu) in the gut of control and antibiotic (Abx)-treated mice showing antibiotic treatment affects the expression levels of copper transporters and the isotopic composition of copper in the colon of mice. Each symbol refers to an individual mouse. Figure from Miller, K.A., Vicentini, F.A., Hirota, S.A., Sharkey, K.A., Wieser, M.E., 2019. Antibiotic treatment affects the expression levels of copper transporters and the isotopic composition of copper in the colon of mice. Appl. Biol. Sci. 116, 5955-5960.
A series of Western blots that show the expressions of the different proteins. Significantly, one can see that the mice treated with antibiotics have very expression of CTR-1, the protein responsible for the transport of copper across the epithelial layer in the proximal colon. Figure from Miller, K.A., Vicentini, F.A., Hirota, S.A., Sharkey, K.A., Wieser, M.E., 2019. Antibiotic treatment affects the expression levels of copper transporters and the isotopic composition of copper in the colon of mice. Appl. Biol. Sci. 116, 5955-5960.
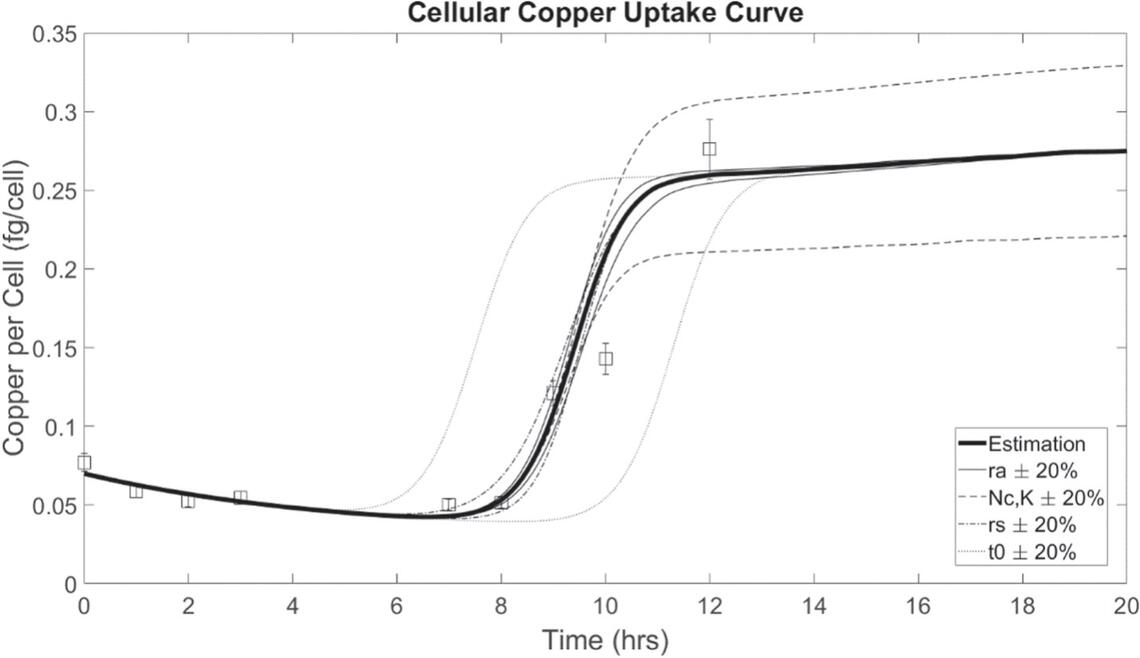
Aaron Wilkins (MSc, 2017 – 2020) employed computational models in combination with isotope dilution mass spectrometric measurements of copper concentrations in the cells of Saccharomyces cerevisiae to explore the dynamics of changes in copper concentration in the cells during growth. Aaron found that a model based on conservation of mass was presented as a system of equations upon which to develop. This system of equations was developed to include an active transport term that describes a homeostatic concentration that cells actively maintain through negative feedback, and with a delayed activation, the model was more accurate at predicting the experimental data. The hypothesis and dynamic model derived in this work provide a novel framework that may be applied to additional metals or used to describe other transport mechanisms in biological systems.
Water is a significant medium for the movement of metals from the geosphere to the biosphere and an important area to study metal transport and incorporation processes. Zinc is an essential micronutrient in the growth of phytoplankton in the ocean and the concentrations and isotopic compositions of Zn in ocean water exhibit trends with depth that suggest that uptake of metals is mediated by biological activity. One critical consideration is to characterize the bioavailable iron and zinc in the water column and constrain the extent of isotopic redistribution resulting from metal uptake and metabolism. Fwziah Mohamed (PhD, 2015-2020/05) is working with Dr. Andrew Bowie and Dr. Bernadette Proemse (UTAS, Australia) on ocean water and sediment samples collected from the Southern Ocean in the vicinity of Heard and McDonald Islands. The concentration of total dissolved iron and zinc in these samples open ocean is typically in the nanomolar (0.1-10 nmol/L) range and only 1 L volumes of sample are available to analyze both Fe and Zn, which places extreme demands on our sample preparation and analytical methods.
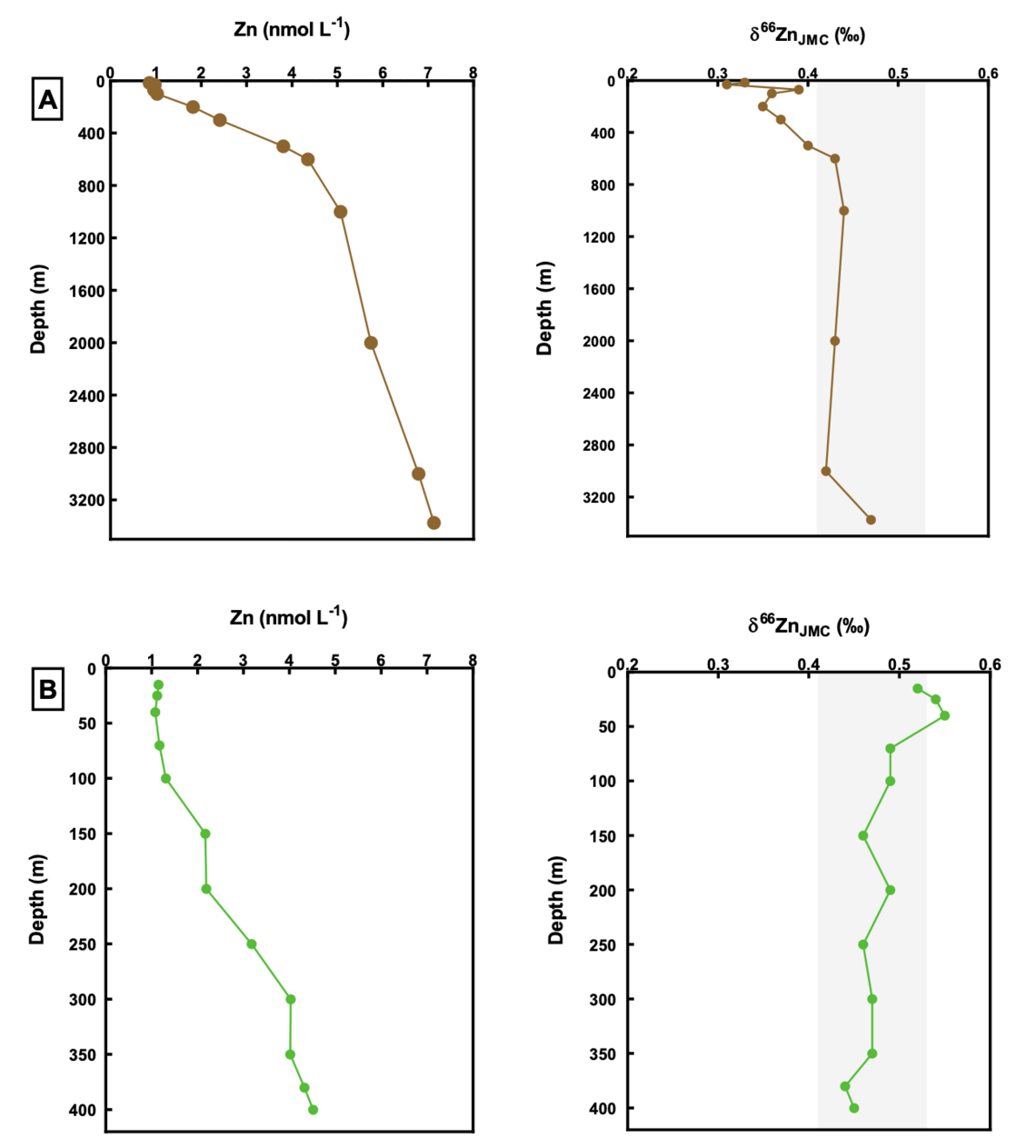
Depth profile of Zn concentrations (nmol/L) and isotopic compositions relative to Lyon JMC of seawater. Samples collected at: A) off the Kerguelen Plateau with the deepest sample collected at 3375 m depth. B) on the Kerguelen Plateau with the deepest sample collected at 400 m depth. The shaded bands show the average δ66Zn value in the deep ocean of +0.47 ± 0.06 ‰.