Research Overview
Calcium Isotope Analysis in Connection to Osteoporosis
The research focus is the investigation and interpretation of calcium isotope abundances for understanding processes in calcium metabolism that can lead to diseases like osteoporosis and osteopenia. In the near term this work will investigate how changes in the metabolism of calcium can affect the distribution of calcium isotopes in living systems. In the long term, the knowledge acquired may extend into clinical practice and ultimately yield benefits to patients suffering from these diseases.
Currently, no non-invasive or inexpensive test is available to continuously monitor bone density or calcium levels in the body. However, there is evidence to show that measurement of calcium isotope ratios in blood and urine can be used as a biomarker for the diagnosis of osteoporosis. In addition to the blood, bone, and urine, one can investigate Ca isotopic compositions and concentrations in hair and nails. We propose that calcium isotope ratios in hair and nails might be used as a new biomarker for the diagnosis of osteoporosis.

Schematic of calcium flow in the body. Figure provided by Dorothy Walls (MSc 2020-2023).
Using Lead-210 Measurements to Quantify Radon Exposure
Though often falsely treated as purely a “smoker’s disease”, radon exposure the second leading cause of lung cancer. Due to poor ventilation systems in modern homes, radon originating from the soil is able to reach high concentrations causing greater exposure for inhabitants. An accurate test for lifetime radon exposure in biological tissue is necessary to provide reliable data for non-smokers in lung cancer screenings. We propose that this can be done by quantifying the amount of 210Pb in readily available biological tissues. This is because 222Rn decays to produce 218Po which then decays to produce 210Pb, which is known to build up in soft keratinizing tissue such as nails. Toenails are ideal target materials since they are disposable tissues and acquiring them is a simple and noninvasive process.
The goal of this project is to determine the most practical way to quantify the lead within the sample. Measurement of the lead isotopes are done by isotope dilution mass spectrometry (IDMS) so we may surpass the sensitivity of current methods. We aim for this method to be able to detect 210Pb present on the order of 10-15 grams of the element. This method of determining lifetime radon exposure marks a fundamental change in thinking in the field, as existing methods for determining radon exposure are based on counting the individual decays of 222Rn. The success of this project will allow for results of high precision and accuracy to be produced simply, cheaply and quickly.
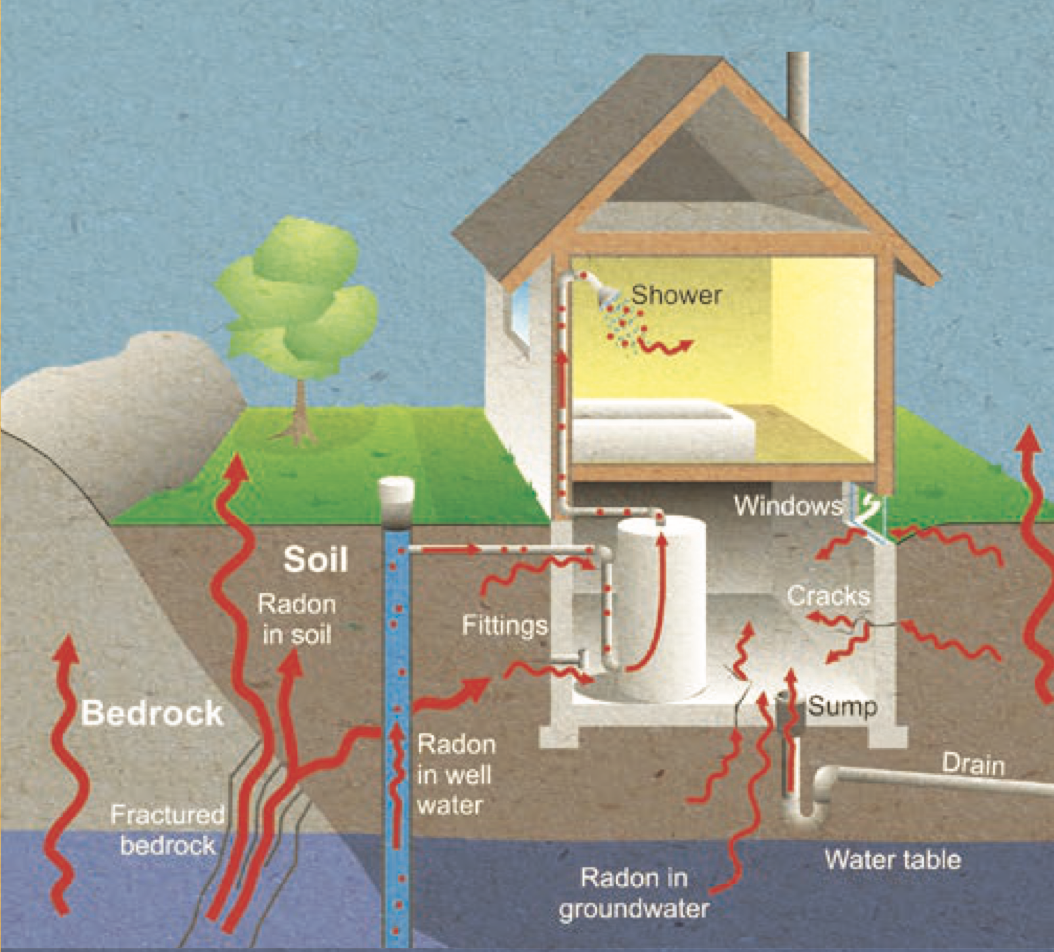
Paths for radon to enter a home. Image from National Resources Canada 2008, courtesy of the Geological Survey of Canada.
Copper and Zinc in Living Systems
Copper and zinc play critical roles in biochemical and geochemical processes. Disruptions in the delicate balance among these elements in living systems are implicated in diseases such as Alzheimer’s, Parkinson’s, and cancer. Often these elements interact in cooperative or antagonistic fashion in biogeochemical processes. The challenge is to gain insights into the physical and chemical mechanisms affecting these elements in living systems and thus understand how these metals are processed in the geosphere and biosphere. Careful quantification of the redistribution of an element’s isotopes provides valuable insights into processes on an atomic scale. Our goal is to understand why changes in the isotopic composition of Cu and Zn occur and exploit this knowledge to understand the role of these elements in biogeochemical interactions. The objectives of the research program will be realized by (1) development and deployment of stable isotope abundance measurements to uncover the distribution and variability of isotopic abundances in biologically significant systems; (2) identification of critical receptors and interactions where changes in isotopic composition occur; and (3) understanding physical and chemical processes responsible for isotopic variations integrating both experimental and theoretical approaches.
Molybdenum in Ground and Surface Waters
Water is a significant medium for the movement of metals from the geosphere to the biosphere and an important area to study metal transport and incorporation processes. In northern Alberta, the extraction of hydrocarbons from oil sand deposits uses water from the Athabasca River as well as recycled water that has come into contact with oil sands, or so-called Oil Sands Produced Water (OSPW). A new technology is being explored whereby petroleum coke, a by-product of the extraction of crude oil from the oil sands, is used to treat OSPW and remove polyaromatic hydrocarbons from the water such that water can be returned to the river. However, the treatment also leads to an increase in pH and some heavy metals including vanadium and molybdenum in treated water. There are many natural sources of heavy metals to the environment such rock weathering, volcanic activity, or natural bitumen seeps like those found in the Athabasca. Therefore, it is vital to distinguish between the natural and anthropogenic inputs so that appropriate procedures can be put in place to minimize human impacts in the region. The ability of isotope abundance data to provide information on the source and history of the material allows this method of analysis to provide unique information.
Schematic illustrating the transport network that is modelled by MCMC algorithms. Figure provided by Aaron Wilkins (MSc 2017-2020/05).
Isotope Metallomics as an Emerging and Powerful Diagnostic Tool
Diseases can lead to subtle changes in the stable isotopic composition of essential elements such as calcium, iron, copper and zinc in living systems. Even though the relative variations in stable isotopic composition are subtle, they are significant, robust and measurable, when using appropriate high-precision mass spectrometry methods (e.g., MC ICP-MS) and calibration strategies. The great potential of the stable isotope method lays in the detection of various diseases at an earlier stage and with less invasive measures than conventional methods. However, in order to apply this stable isotope method for elements such as calcium, iron, copper and zinc in the clinical setting, reliable and precise high-throughput isotopic analysis procedures must first be developed – that do not exist yet. Current procedures for high-precision isotopic analysis are time-consuming, labour-intensive and have high instrument running costs. Analyte purification is the main bottleneck for high-throughput isotopic analysis with its low sample throughput. Automation is key here – and this is exactly where the approach of this project comes in.
In recent years, commercial automated chromatographic systems for analyte purification have been developed – such as the prepFAST-MCTM (Elemental Scientific) – that are fast and overall very efficient by unattended operation, more reproducible flow rates and elution volumes, and lower procedural blanks. To date, automated analyte purification has been of limited use for mainstream biomedical applications because of low matrix tolerance or because automation is interrupted by evaporation and resin exchange steps in previously developed procedures. Consequently, in this project we will utilize the prepFAST-MCTM and develop automated two-stage separations procedures – as required for the isotopic analysis of essential mineral elements (e.g., iron, copper, zinc).
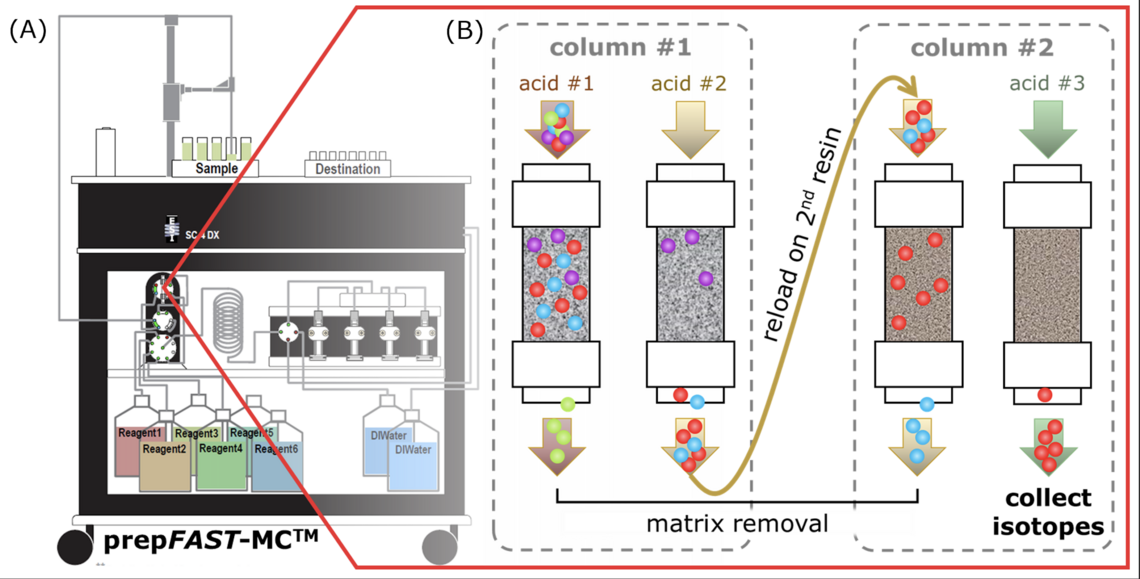
Panel A. Schematic of the prepFAST-MCTM system by Elemental Scientific. Panel B. Simplified illustration of a two-stage separation procedure – as it is intended to be implemented at the prepFAST-MCTM system. Reference prepFAST-MCTM illustration: Elemental Scientific.
Publications
Quantitative assessment of the radon (222Rn) decay product 210Pb in human toenails as a sensitive measure of personalized long-term radon gas exposure history
Kerri A. Miller, Dustin D. Pearson, Sophie C. Pett, Michael E. Wieser, Aaron A. Goodarzi
Lung cancer mortality can be lowered through early-diagnostic screening of people demonstrating a ≥1.5 % 6-year risk of tumor development. However, many who develop lung cancer are ineligible for screening (~40 % of Canadian patients) as they have insufficient tobacco smoking history. Tools to assess individual lung cancer risk based on exposure to other prevalent environmental carcinogens such as radon (222Rn) gas are lacking. Here, we explore ultrasensitive quantification of the 222Rn decay product 210Pb in toenails (n =39) as an indicator of personalized, long-term radon exposure history. Toenail cuttings from adults inhaling elevated indoor radon in their primary house (average radon =354.9 Bq/m3) over a mean of 26.5y (equating to 427 mSv radiation dose) contained 0.298 femtograms of 210Pb per nanogram of stable Pb. By contrast, only 0.075 femtograms 210Pb per nanogram Pb were detected in toenails from low radon exposure controls (28.4 Bq/m3 over 22.5y equating to 22.8 mSv) – a 397 % difference. Notably, elevated radon decay products (0.245 femtograms of 210Pb per nanogram Pb) persisted in toenails from highly radon-exposed people (545.6 Bq/m3 over 18.5y equating to 283 mSv) who, up to 6 years prior to toenail collection, had mitigated their primary residence to reduce radon (post-mitigation radon =28.1 Bq/m3). No differences were detected on the basis of sex, age, tobacco smoking history, or 210Pb-rich game meat consumption. These data suggest that toenail 210Pb/Pb isotope (amount) ratios show promise for evaluating individualized retrospective radon dosimetry history – an approach that may become helpful to assess non-tobacco lung cancer risk in the future.

