
Carbocations and Free Radicals
The structure, reactivity and stability of organic carbocations and free radicals
Carbocations
Carbocations are organic compounds with a tricoordinated, sp2-hybridized, carbon atom with a formally empty 2p orbital. They are positively charged electron deficient compounds with often very fluid structures. Very few are stable isolable entities; they are usually postulated as reactive intermediates in SN1 and E1 mechanisms.
The most interesting features of carbocations are their nonclassical structures and ready conformational interconversions. The first are very amenable to ab initio electronic structure calculations, while the second are also susceptible to experimental strong acid NMR techniques. In the 1980a and 1990s, my group had a fruitful collaboration with the Sorensen group, beginning with his seminal discovery in 1980 of novel cyclohexyl cations which can be described as hydride-bridged dications [T. S. Sorensen, et al., JACS, 1981, 103, 588 – 596; T. S. Sorensen, et al., JACS, 1981, 103, 597 – 604]. The empty 2p orbital has a tendency to delocalize electrons from nearby s bonds and will distort from a planar structure to maximize p-type overlap [T.S. Sorensen et al., JACS, 1989, 111, 9024 - 9029]. This is dramatically illustrated in the structure of the 2-adamantyl cation shown at right.
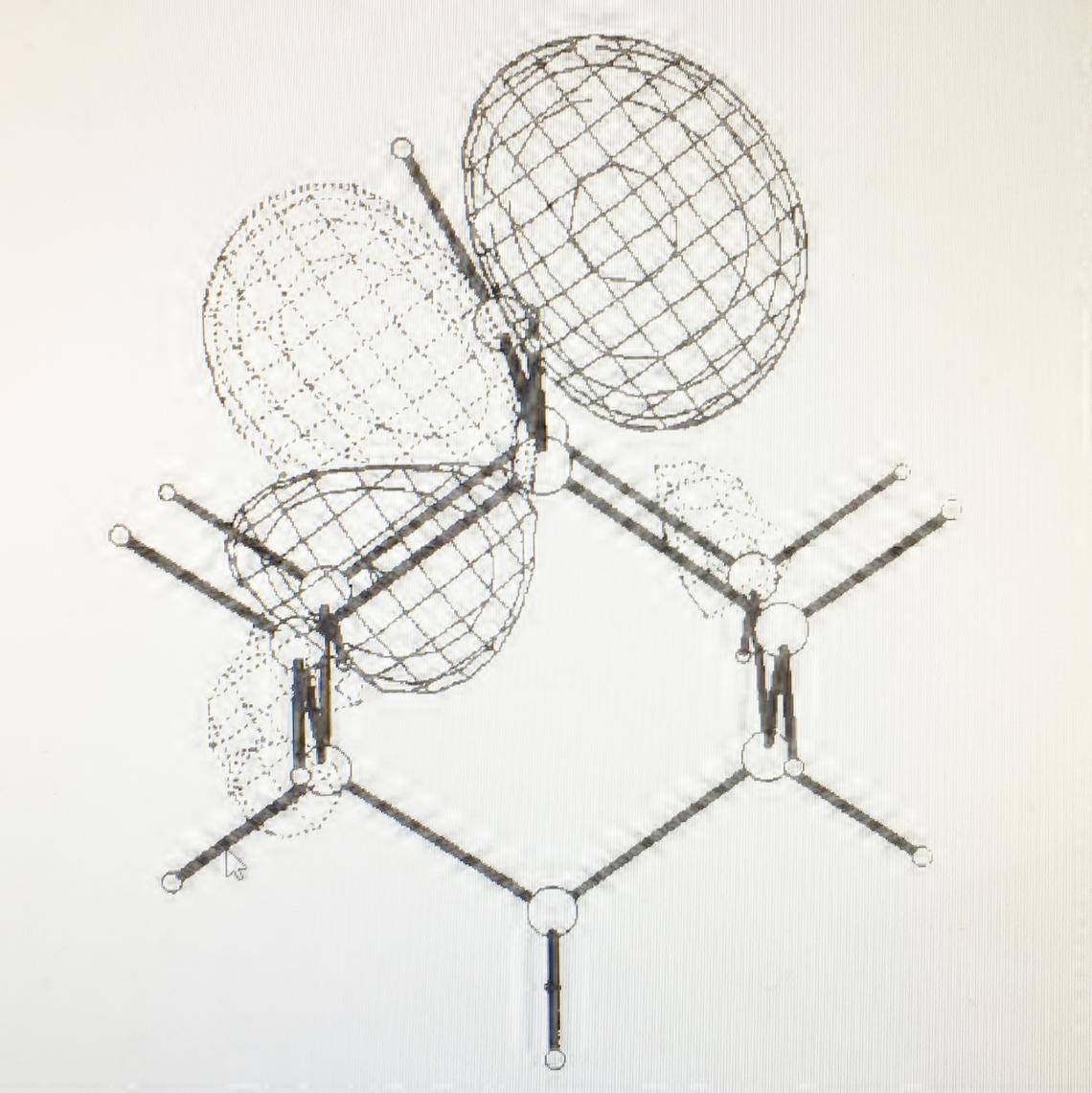
Computed structure of the 2-adamantyl cation, showing distortion from nonplanar geometry to maximize orbital overlap with adjacent C-C bonds. The lowest unoccupied molecular orbital is shown.
Organic Free Radicals
The central feature of organic free radicals is an sp2 hybridized carbon atom with a singly occupied 2p orbital.
For a variety of theoretical and technical reasons, computation of open shell systems is difficult at the Hartree-Fock level. Post-HF methods, e.g., UMP2 with a large basis set, were required to yield reliable structures, energies and thermodynamic functions. It became very much easier with the advent of Density Functional Theory (DFT) in the early 1990s. Nevertheless, I enjoyed a very fruitful collaboration in the 1980s with the late Eugene (Gene) Tschuikow-Roux and his student Yonghua Chen, investigating the structures of a variety of 2- and 3-C hydrocarbons with varying degrees of fluorination. Gene was a kineticist whose modus operandi was gas-phase shock tube, which yielded experimental data in the gaseous phase, ideal for computations, since the effects of solvation were difficult to accommodate in those days. The structures of mono- di- and trifluoroethyl radicals are shown at right [Y. Chen, A. Rauk, and E. Tschuikow-Roux, J. Chem. Phys. 1990, 93, 6620 – 6629].
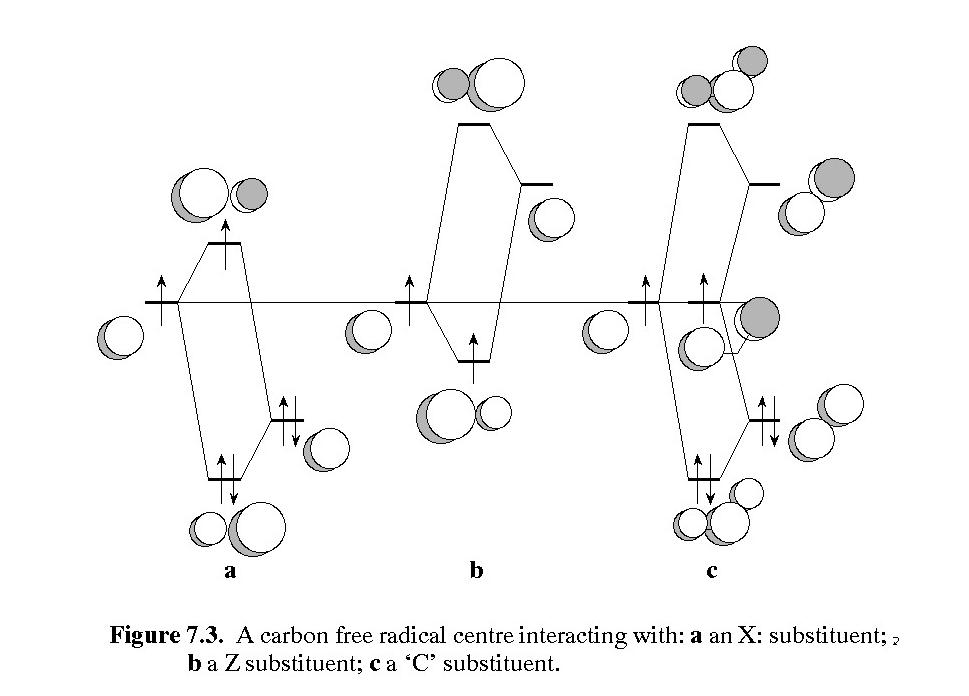
Stabilization of carbon free radical by an electron donor (left), an electron acceptor (middle), and by the captodative effect (right) - image from "Orbital Interaction Theory of Organic Chemistry, 2nd Ed.", John Wiley & Sons, 2000.
Alpha-C Radicals in Peptides
As the orbital interaction diagram above shows, extra stabilization of a C-centred radical, called captodative stabilization, ensues if the radical is substituted by at least one p-electron donor (O, N, double bond, etc), and one p-electron acceptor (-NO2, C=O, nitrile, double bond, etc). While a normal C-H bond dissociation enthalpy (BDE) is above 400 kJ/mol (CH4 440), much lower values of BDE are measured and calculated for captodative systems:H2NCH2CH=O 320; H2NCH2BH2 333, CH2=CHCH2CH=CH2 250). For maximum stabilization, the p-systems of the donor, acceptor and C. must be coplanar. We realized in the late 1990s that the a-CH bond common to all amino acid residues and proteins may similarly be weakened by the captodative effect of the flanking amide groups, one of which presents a carbonyl group and the other of which presents the NH group. The carbonyl group of an amide is a weak p-acceptor and the NH group is a weak p-donor, so it was not clear how much captodative stabilization actually ensues. The question is important in the biological context because removal of the a-CH hydrogen atom constitutes oxidative damage to the peptide/protein. Oxidative damage is usually repaired by transfer of an H atom from glutathione (GSH), the endogenous reducing agent present in millimolar concentrations. Repair may not be possible if the a-CH bond is weaker than the S-H bond of GSH, 367 kJ/mol. The graphic at the right represents the results of a careful theoretical investigation of the BDEs of all peptidic a-CH bonds (A Rauk , D Yu, J Taylor, G V Shustov, D A Block, D A Armstrong, Biochemistry, 1999, 38, 9089-9096. doi: 10.1021/bi990249x). The figure on the right displays the -C-H bond dissociation enthalpy (BDE) of each of the amino acid residues modeled as peptides, (red dots). The black horizontal line is the experimental BDE of the S-H bond in glutathione and cysteine. With one exception, all of the a-C-H bonds are predicted to be weaker than the S-H bond!
The Radical Model of AD

The model postulates that a long-lived a-C Gly radical can enter from the aqueous environment into a hydrophobic interior of an unsaturated lipid bilayer and initiate lipid peroxidation by abstracting a CH2 hydrogen atom to form a stable pentadienyl radical. The a-C Gly radical is generated by an oxidized Met S radical, which in turn is generated by Cu(II) ot Cu(I) bound to A-beta.
The Radical Model of AD
Through a series of computational investigations, we discovered that captodative stabilization is not possible in a-helical secondary structure, but b-sheet secondary structure is planar enough and flexible enough to stabilize an a-C-centred radical. However, a b-sheet has the sidechain of the residue perpendicular to the average plane of the sheet; the a-C-H bond lies in the plane, pointed toward the adjacent beta strand and is inaccessible to an attacking free radical. The requirements of the transition structure for H-atom abstraction is that the abstracting radical also lie in the plane, an impossible situation. As a consequence, only a Gly residue in a beta sheet is susceptible to damage since its upward-pointing “sidechain” is a hydrogen atom. Since beta amyloid has six Gly residues, including four in reasonable proximity to Met35, namely Gly29, Gly33, Gly37 and Gly38, and since beta amyloid assembles in beta sheet secondary structure, we showed that an oxidized Met, MetS+, could reach across to a Gly residue in an adjacent b-strand to abstract the aC-H atom, and is energetic enough to do so. However, a bound Cu(II) ion could not oxidize a Met S atom unless it was stabilized by proximity to another Met. The two major difficulties with the Radical Model are that the Cu(II)2+ is bound to two or more of the His residues, His5, His13 and His14 which are far removed from the Met35, and secondly, beta amyloid only has one Met, not the required two. These difficulties were overcome by the observation that the reduced form of copper, Cu(I), can generate hydroxyl radicals by reaction with molecular oxygen, and hydroxyl radicals can oxidize a single Met S atom.
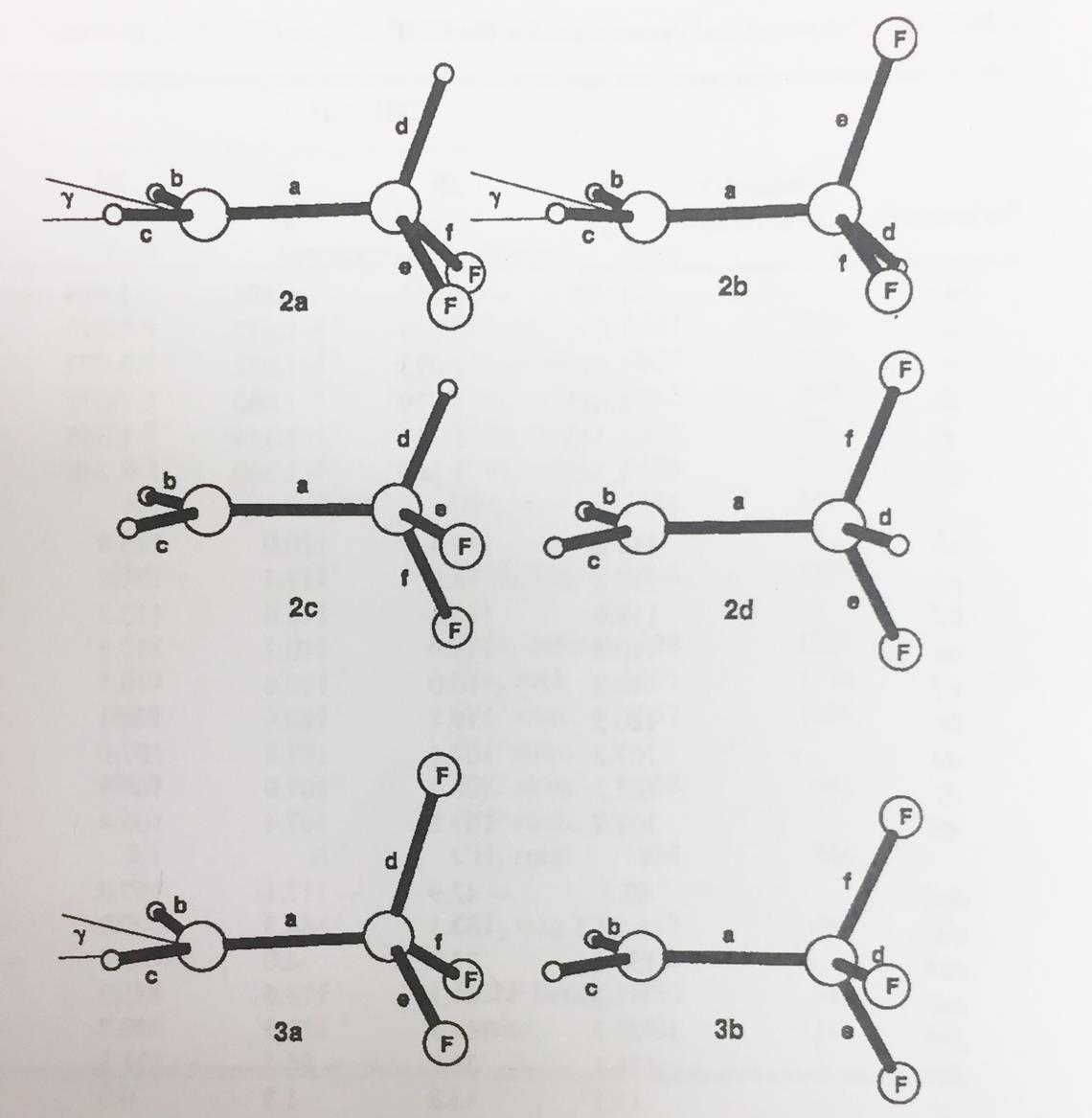
The UHF/6-31G(d) structures of 2-fluoro-, 2,2-difluoro-, and 2,2,2-trifluoroethyl radicals.
Stabilization of Free Radicals
Throughout the 1990s, we had a very fruitful collaboration with the late David Armstrong and a shared PDF, Dake Yu, investigating radicals with a more biological relevance. With the advent of DFT and more sophisticated theoretical and computational techniques, answers to more complex questions could be obtained. Orbital interaction theory (OIT) predicts that radicals can be stabilized by delocalizing nearby electrons into the singly occupied 2p orbital of the radical centre (the donor mechanism), and counterintuitively, also by delocalizing the single electron into nearby empty p-type orbitals (the acceptor mechanism). Clearly, since every molecule has within it a source of both occupied and empty orbitals, as do external molecules, including the medium, all radicals should be stabilized by these two mechanisms. While OIT provides only a crude estimate of the extent of stabilization, DFT methods can provide a quantitative number, in the form af an enthalpy of interaction (R. + X -> R.X), or the strength of a bond (R:H -> R. + H.). In some molecules there is the possibility of both donor and acceptor mechanisms operating to an optimum extent, e.g., a C-centred radical with an adjacent amino group and an adjacent carbonyl group. Such radicals experience "captodative" stabilization.
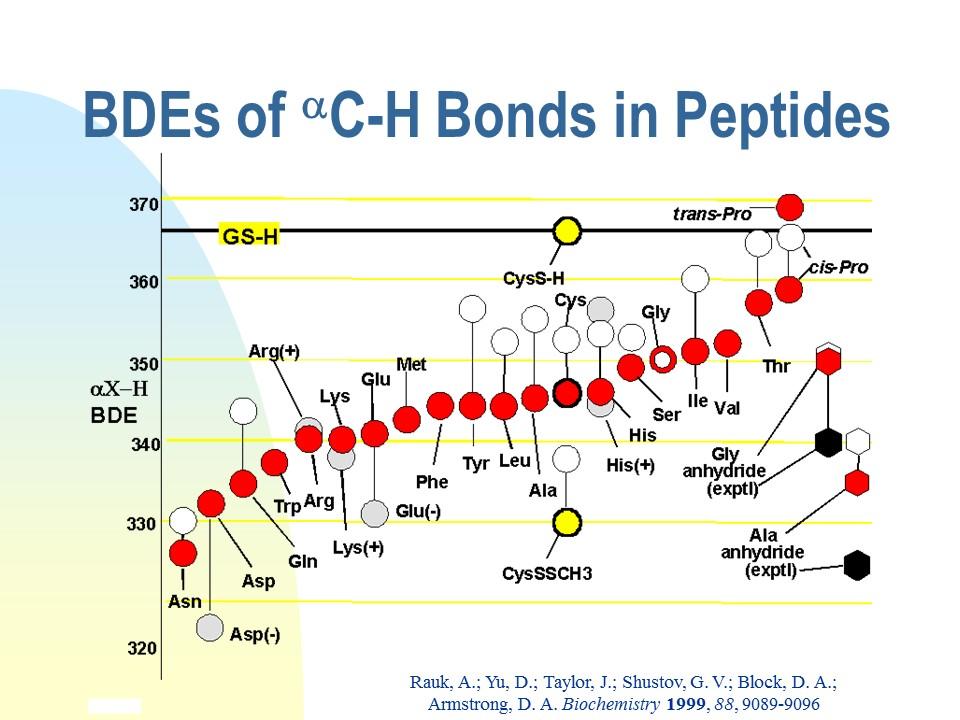
Bond dissociation enthalpies of all of the amino acid peptides.
Oxidative damage to Proteins and Peptides
The predicted result, that the a-C-H bond of most peptides is weak and H atom removal would lead to a stable a-C radical, opened a whole raft of unanswered questions:
- Except for glycine, all amino acids are chiral with the L-absolute configuration. Generation of a planar a-C radical, and subsequent repair by replacement of the H atom, may lead to generation of unnatural D-configuration a-C centres. In fact, D-amino acids are rare in biological systems. Why?
- The predicted maximal captodative stabilization of a-C radical centres requires that all 12 atoms of the two amide groups and the intervening sp2 a-C site be able to be coplanar. Is such a configuration even possible in proteins and peptides?
- Is there literature evidence of protein- or peptide-based radicals?
Indeed, a quick literature search immediately pointed to the beta-amyloid peptide of Alzheimer’s disease as a possible source of radicals. Unexpectedly, Met 35 was also implicated. As was Cu(II), itself an ion with an unpaired electron, i.e., a free radical. Also associated with AD, were brain inflammation and membrane damage by lipid peroxidation. The first step of lipid peroxidation is the generation of a lipid C-radical in the interior of a lipid bilayer, which then picks up dissolved molecular oxygen to form a peroxy radical. Could a captodatively stabilized a-C-Radical of beta amyloid infiltrate a neuronal cell membrane and abstract an H atom from a polyunsaturated -CH=CH-CH2-CH=CH- centre to initiate the lipid oxidation process? How could such a radical be generated and at which residue of beta amyloid?
