Rapid Preparation of Pharmaceutical Samples for Analysis
Increasing throughput and decreasing costs in pharmaceutical drug development is critically important to the industry. As such, any efforts that can reduce the analysis times and increase sample throughput will have a significant impact on the ability of pharmaceutical companies to bring drugs to market faster. For example, low-level impurities that may form during manufacturing and/or product shelf life are of great concern owing to their potential impact on drug quality/efficacy. Due to the low concentrations involved, however, challenging issues arise for quantifying pharmaceuticals in complex matrices. At present, sample preparation involves lengthy, inefficient procedures that are not always effective at extracting target drug analytes either quantitatively and/or rapidly. Also, current methods do not address the long term needs of reducing large volumes of solvent, which contribute to hazardous waste concerns and considerable difficulties in detecting low-concentration drugs. Since modern chromatographic methods used in many of these analysis are relatively rapid, the current sample preparation methods employed are one of the main reasons that laboratories struggle in addressing the need for high throughput analysis. As such, the development of new methods that can rapidly and efficiently extract pharmaceuticals and their impurities in a format directly ready for analysis will have a significant impact on sample throughput in this important routine analytical procedure. Our project is focused on exploring novel sub-critical fluid methods of pharmaceutical sample preparation that are aimed at increasing sample throughput in the industry.
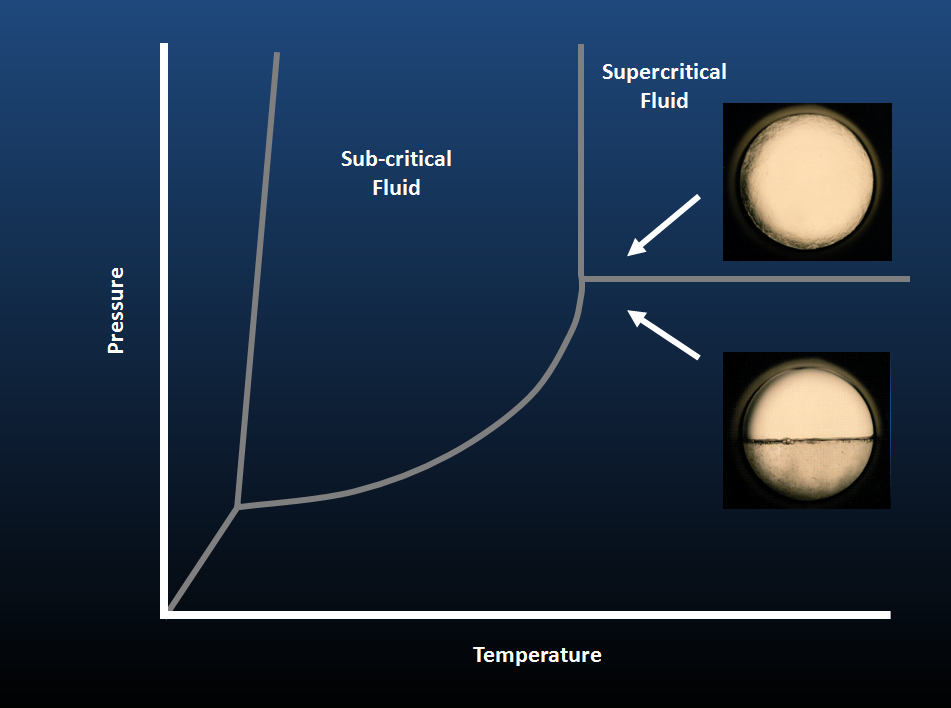
Sub and Supercritical Fluid Extraction (SFE)
Sub and Supercritical fluids are widely used for separations in analytical chemistry. Since they exist under conditions near the critical point of a fluid, they have properties of both a gas and liquid. For example, they have the dissolving power of a liquid but mass transport capabilities approaching those of a gas. Supercritical fluid extraction (SFE) is a proven technology that takes advantage of these beneficial properties and others in sample preparation. For instance, the gas-like mass transport properties of the fluid promote fast analyte diffusion and equilibration. Moreover, the low viscosity of a supercritical fluid allows it to penetrate porous analyte matrices better than a liquid and improve analyte recovery. Also, since it is very compressible near its critical point, supercritical fluid density can be readily controlled through applied pressure. As such, density gradients in SFE can often enhance the extraction selectivity of certain analytes over others that are more soluble under select pressures. Supercritical CO2 is one of the most widely used fluids due in part to its environmental compatibility, inertness, low cost, and mild critical parameters (31oC, 73 atm) relative to many organic solvents. As well, since it decompresses to a gas upon exiting the extraction vessel causing the extracted analyte to accumulate in a readily pre-concentrated form for analysis. In all, these properties often allow fast, efficient and selective SFE extractions to be conducted with supercritical CO2 and a variety of other fluids, without compromising analyte integrity, generating significant waste solvent, or expending enormous time of subsequent evaporation steps.