Research overview
The below figure summarizes different aspects of our applied research. The research encompasses a broad range of objectives with common theme issues involving the production, conditioning and processing of natural gas fluids containing H2S and CO2.
In general, H2S must be removed from sales gas due to its toxicity and to avoid the release of SO2 into the environment upon combustion. Water is removed to avoid flow assurance issues by clathrate hydrate formation or to reduce corrosion by eliminating a liquid water phase. CO2 is removed to increase the caloric content of sales gas and avoid extraneous corrosion.
Summary of Our Applied Research Projects
Summary of Our Applied Research Projects
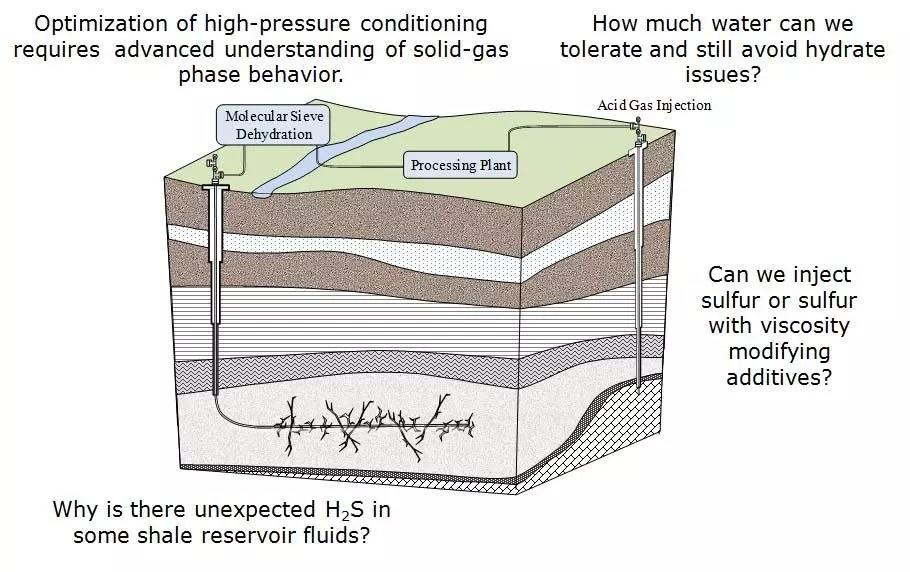
Project 1: The absorptive dehydration or desulfurization of raw production fluids before being transported through gathering lines to gas plants.
Most of the reported adsorption studies involving gases containing H2S have been conducted at low pressures (p < 0.1 MPa) and a few at moderately high pressures (p < 6.9 MPa); however, much higher pressures are applicable to industrial fluids, e.g., reservoir sour gas, pipeline sour gas, producing shale gas and compressed CO2 injectate for carbon capture and storage. Besed on location and cost, many current upstream gas processes use zeolitic molecular sieves for deep dehydration of sour gases. At high-pressures, non-ideal surface coadsorption, chemical reactions and non-ideal chemical potentials can cause selective adsorption to be significantly different than those measured at low pressures. Our laboratory is one of only a few facilities world-wide where such studies can be carried out.
Project 2: The passive dehydration of injectate fluids before being transported to an injection well.
In addition to adsorption, there are other methods for dehydrating injectate fluids (H2S and/or CO2 liquids) such as the passive technique of having the water condense within the coolers and suction scrubbers between multistage compression cycles. Again, there is limited information in this area to predict or estimate how far one needs to dehydrate a liquid acid gas. If an H2S and CO2 fluid contains too much water, there is a risk of forming clathrate hydrate solids from a single phase during transportation to the injection site. Normally, laboratory hydrate solid formation conditions are measured in the presence of three phases (hydrate phase, non-aqueous fluid phase and liquid water phase). For a sub-saturated fluid such as a passively dehydrated injectate, a temperature drop could cause hydrate to form without a pre-existing liquid water phase (sub-saturated region). This research involves studying the melting of hydrates through variety of high-pressure techniques.
Project 3: The pumping or injection of liquid elemental sulfur.
Elemental sulfur is recovered from fuels and other hydrocarbons to reduce the environmental impact of SO2 release. There are many areas where recovered sulfur is stranded from markets and must be stored for long periods of time, e.g., the Alberta Oil Sands. After transforming the recovered elemental sulfur into a liquid phase, containing H2S and/or amine degassing catalysts, it often is pumped from liquid sulfur pits or holding tanks. The injection of liquid sulfur into depleted reservoirs may be a safer alternative for long term sulfur storage; however, the viscosity under various shear rates must be well understood before attempting injection.
Project 4: The volumentric properties of wet acid gases.
Optimizing high-pressure conditioning and sweetening processes, requires a detailed understanding of the thermodynamic mixture properties. For example, several research groups focus on the volumetric changes for water, when a solute is introduced. CO2 and H2S fluids are often injected as a dense phase (supercritical or liquid), where volumetric differences are important for modelling the higher pressure phase behavior and chemical reactions. This project looks at the change in the volumetric properties of an acid gas, upon introducing water as a solute. These studies require a very high-accuracy densimeter which has been built in-house.
Project 5: Sources of H2S in shale gas fluids.
This new research area aims to identify the source of H2S in shale fluids. Often producers encounter small amounts of H2S in shale fluids which are expected to be sweet. Dealing with small amounts of H2S can be problematic, especially if it was not expected when the production equipment was put in place. This research looks at the various possible sources of H2S, by exploring high-pressure chemistry which is known to generate to H2S in other fuel sources/production schemes.
