Research
Research Areas
Chemical reactions of metal-based drugs with S-containing biomolecules
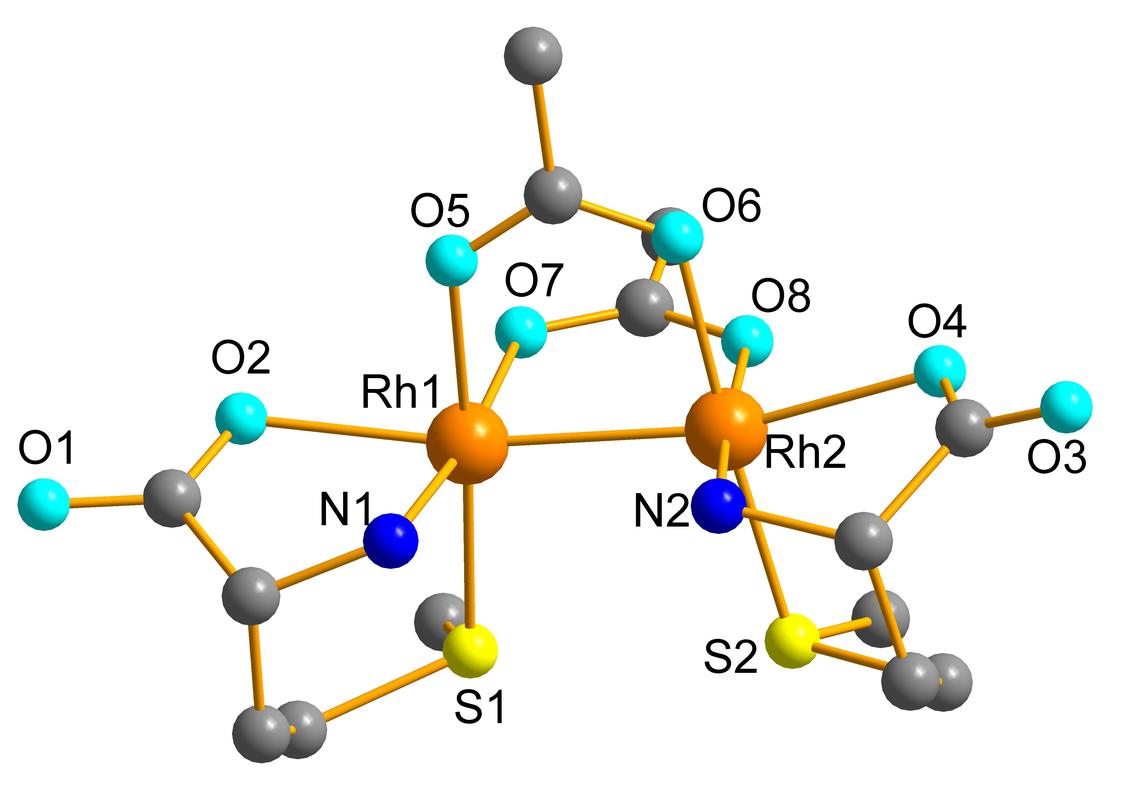
Anticancer platinum-containing drugs such as cis-platin, [Pt(NH3)2Cl2], are widely used comprising 50% of all chemotherapeutic drugs in clinical use. However, their lack of specificity causes severe toxic side effects, triggering cell death of both cancerous and healthy cells. Also, development of cellular resistance toward these drugs may restrict the treatment. Therefore, complexes of other metal ions such as Au, Ti, Rh, Re and Ru are under investigation as alternative drugs for treating cancer. However, these metal-based drugs also interact with other biomolecules such as proteins and enzymes, and only a small percentage reach their cellular target to disrupt its replication mechanism.
Our group is interested in how metal complexes that are potential candidates for cancer treatment can interact with sulfur-containing biomolecules, such as the amino acids cysteine and methionine, and the tripeptide glutathione, as models for their interactions with S-containing proteins and enzymes. In collaboration with Professor Shemanko group, we also investigate on how such interactions can impact their cytotoxicity.
References
A. Enriquez Garcia and F. Jalilehvand*, J. Biol. Inorg. Chem. 2018, 23, 231 – 239 (Abstract)
M.S. Capper, A. Enriquez Garcia, N. Macia, B. Lai, J‑B. Lin, M. Nomura, A. Alihosseinzadeh, S. Ponnurangam, B. Heyne, C.S. Shemanko and F. Jalilehvand*, J. Biol. Inorg. Chem. 2020, DOI: 10.1007/s00775-020-01798-9 (Abstract)
Mapping distribution of elements in cells using synchrotron light
Synchrotron facilities equipped with state-of-the-art optics can generate intense X-ray radiations with high resolution, suitable for in-situ elemental mapping in micron or sub-micron levels. We use this powerful technique, known as synchrotron-based X-ray fluorescence miscroscopy, to probe how metal-based drugs accumulate in cells and to monitor how their reaction products with S-containing species can impact their cellular uptake. The cell culture procedures are carried out in collaboration with Professor Shemanko group.
References
A. Enriquez Garcia, B. Lai, S. Gopal Gopinathan, H.H. Harris, C.S. Shemanko and F. Jalilehvand*, Chem. Comm. 2019, 55, 8223 – 8226 (Abstract)
M.S. Capper, A. Enriquez Garcia, N. Macia, B. Lai, J‑B. Lin, M. Nomura, A. Alihosseinzadeh, S. Ponnurangam, B. Heyne, C.S. Shemanko and F. Jalilehvand*, J. Biol. Inorg. Chem. 2020, DOI: 10.1007/s00775-020-01798-9 (Abstract)
Complex formation of heavy-metal ions with small thiol-containing molecules
Accumulation of heavy metal ions in the environment causes adverse health effects. Uptake via food, drinking water and air of Hg(II), Pb(II) and Cd(II) can affect human metabolism by blocking enzymatic functions. “Soft” metal ions have high affinity for thiol (-SH) groups, in e.g., the amino acid L-cysteine, the cysteine-rich protein metallothionein, and the tripeptide glutathione (Glutamyl-Cysteine-Glycine), which is the most abundant cellular thiol in the body and has important in vivo functions for protection against heavy metal ions. Thiol-containing drugs, e.g. D-penicillamine (3,3´-dimethylcysteine) and N-acetylcysteine, are clinically used for heavy metal detoxification.
We apply a combination of different spectroscopic methods such as multi-nuclear NMR, X-ray absorption (EXAFS) and vibrational spectroscopy to investigate the coordination of such metal ions to glutathione, cysteine and its derivatives, to elucidate the structure of their complexes, and to evaluate the species formed in aqueous solution. Information on structure and bonding of heavy metal complexes with such thiol-containing small molecules can assist design of new chelating agents/ drugs with improved efficiency for detoxification.
References
N. Sisombath, F. Jalilehvand*, A.C. Schell and Q. Wu, Inorg. Chem. 2014, 53, 12459 – 12468 (Abstract)
V. Mah and F. Jalilehvand,* Chem. Res. Toxicol. 2010, 23, 1815 - 1823 (Abstract)
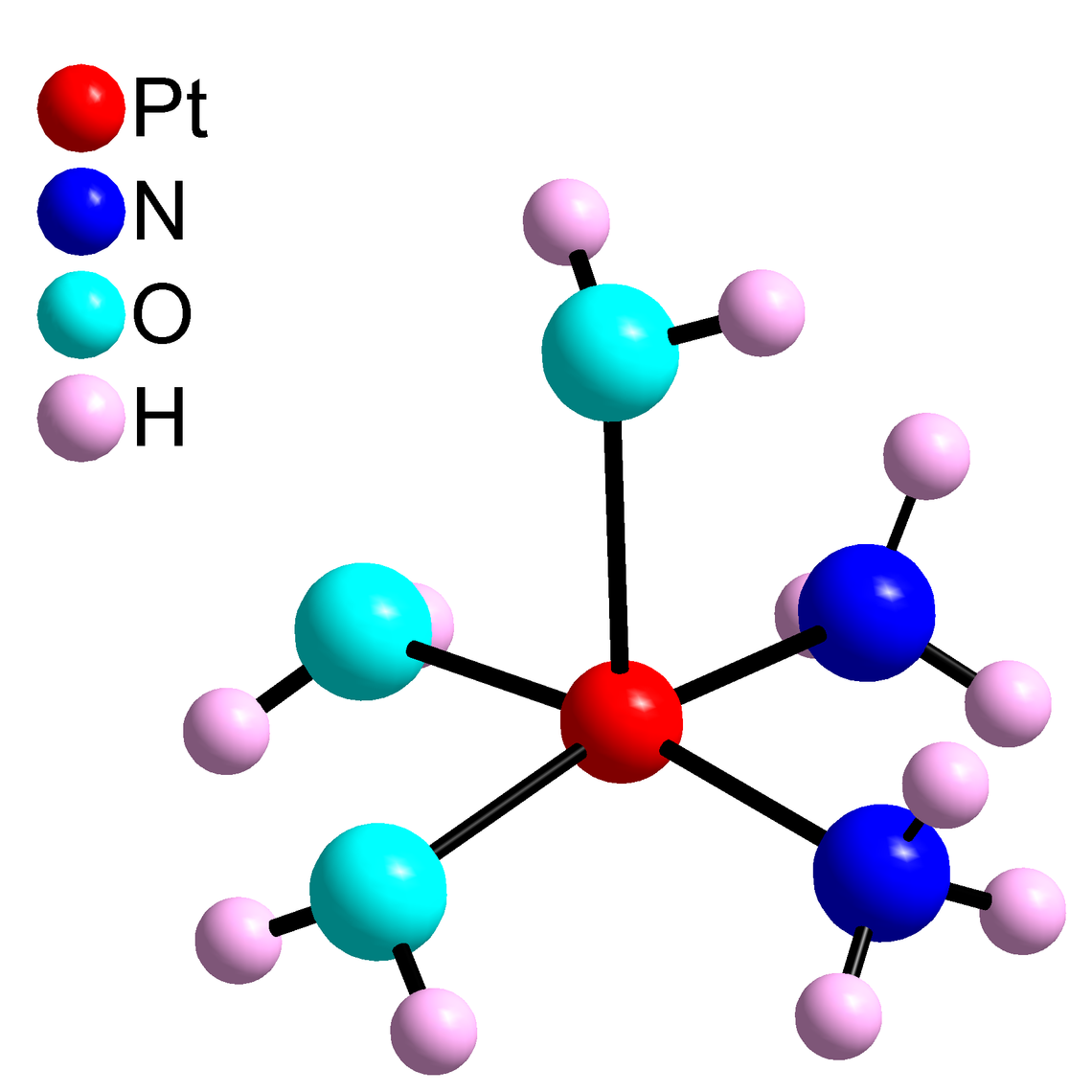
Structural analysis of solvated ions with biological relevance
We also investigate the structures of solvated ions with biological relevance, such as the [Pt(H2O)n]2+ ion and cis-[Pt(NH3)2(H2O)m]2+ complex, as one of the active forms of the anti-tumor drug cis-platin inhibiting cell division. When entering a cellular environment, the drug cis-platin, cis-[Pt(NH3)2Cl2], is activated by substitution of one or both chloro-ligands with water molecules. Structural information of the hydration products of cis-platin is essential for understanding its reactions.
References
F. Jalilehvand* and L. J. Laffin, Inorg. Chem. 2008, 47, 3248- 3254 (Abstract)
L. Kocsis, J. Mink,* F. Jalilehvand, L. J. Laffin, et al., J. Raman Spectrosc. 2009, 40, 481 - 490 (Abstract)
Sulfur speciation in waterlogged wood of historical shipwrecks
We applied sulfur K-edge XANES, for the first time, to marine-archaeological wood samples, and could reveal the cause of a severe problem for such artifacts. Sulfate salt formation had been observed on the 17th century shipwreck of the warship Vasa in Stockholm, Sweden. The sulfur XANES spectra showed that a large amount of elemental sulfur, stored in the moist wood, was being oxidized to sulfuric acid, causing wood deterioration. We also studied other artifacts and shipwrecks such as the Mary Rose (England), the Batavia (Western Australia) and the Bremen Cog (Germany), to study how general the sulfur problem is, and the role of sulfides such as pyrite in this process.
This project has been completed.
References
M. Sandström*, F. Jalilehvand, et al., Nature, 2002, 145, 893-897 (Abstract)
M. Sandström*, F. Jalilehvand, et al., PNAS 2005, 102, 14165-14170 (Abstract)